Background: Although it is known that the annualized relapse rate (ARR) in MSers changes as the disease progresses, in the design and analysis of trials in RRMS constant ARRs are assumed.
Objectives: This paper aims to assess time-patterns of trial ARR by conducting a systematic review of randomized, placebo-controlled trials in RRMS.
Results: A total of 52 trials was identified. Out of these, information on the time-dependence of trial ARR could be extracted from 13 trials. The ARR was by 25% (p = 0.0005) and 40% (p < 0.0001) higher in months 1-12 compared with months 13-24 for placebo and active treatments, respectively. Consequently, the treatment effects were by 13% (p = 0.23) larger in the second year compared with the first year. Within the first year of follow-up the ARR was by 4% (p = 0.75) and 23% (p = 0.06) higher in months 1-6 compared with months 7-12 for placebo controls and active arms, respectively.
Conclusions: Trial ARR decreases during a trial in RRMS, which is in line with epidemiological findings and has implications for design and analysis of future trials. The observed decrease in trial ARR might be at least partially explained by regression to the mean. Individual patient data analyses are warranted.
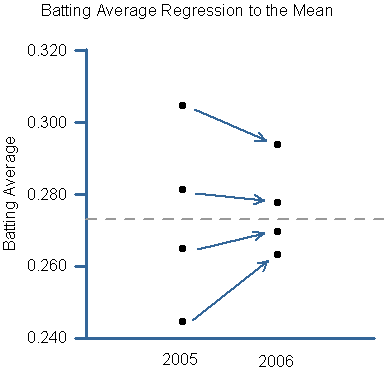
"Regression towards the mean refers to the phenomenon that if your select MSers for trials based on disease activity their disease is likely to become less active during the course of the trial, which is what this study shows. Therefore there was a 25% change in ARR rate in placebo. A change of 40% in drug-treated group is because there is a treatment effect of the drug to slow ARR. Is this important? Not really as clinical trials are placebo-controlled and randomised, this allows comparisons across the two arms; so what happens to one arm of the study will happen to the other arm and any meaningful difference should be due to a treatment effect. This is why it is so difficult to assess what happens in open-label observational studies are individual cases."
Additional reading: regression to the mean